Press Release
Cleo Breast has received CE marking under the new IVDR regulation.
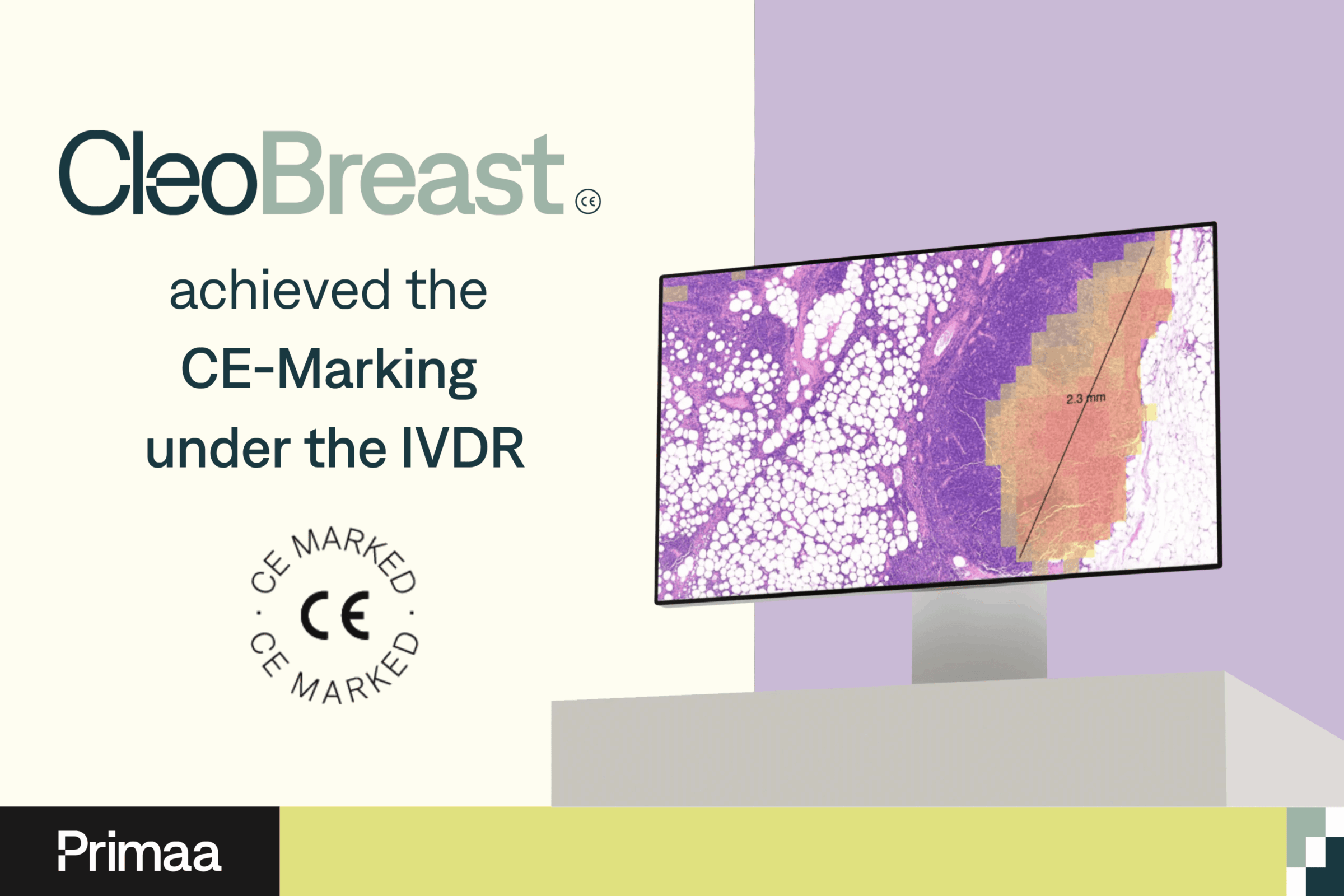
- Primaa, a leader in Artificial Intelligence (AI) solutions applied to pathology, announces that its software Cleo Breast has obtained CE marking under the new European Regulation (EU) 2017/746 IVDR (In Vitro Diagnostic Regulation).
- The first version of Cleo Breast has been self-certified since 2021. Following major product developments, it became essential to obtain the new regulation. Obtaining IVDR certification marks a key strategic milestone to ensure the sustainability and widespread adoption of the tool across Europe.
- The tool now includes a mitosis detection feature, an important biomarker in the diagnosis of breast cancer.
- The IVDR marking positions Primaa among the first European companies to have successfully complied with this new regulation, considered one of the most demanding in the world in the field of medical diagnostics.
AI, a strong support for the diagnosis of breast cancer
Breast cancer is the most common cancer among women, with nearly 2.3 million new cases diagnosed each year worldwide. By enhancing the precision and reproducibility of diagnoses, Cleo Breast helps improve early detection and optimize therapeutic management of patients.
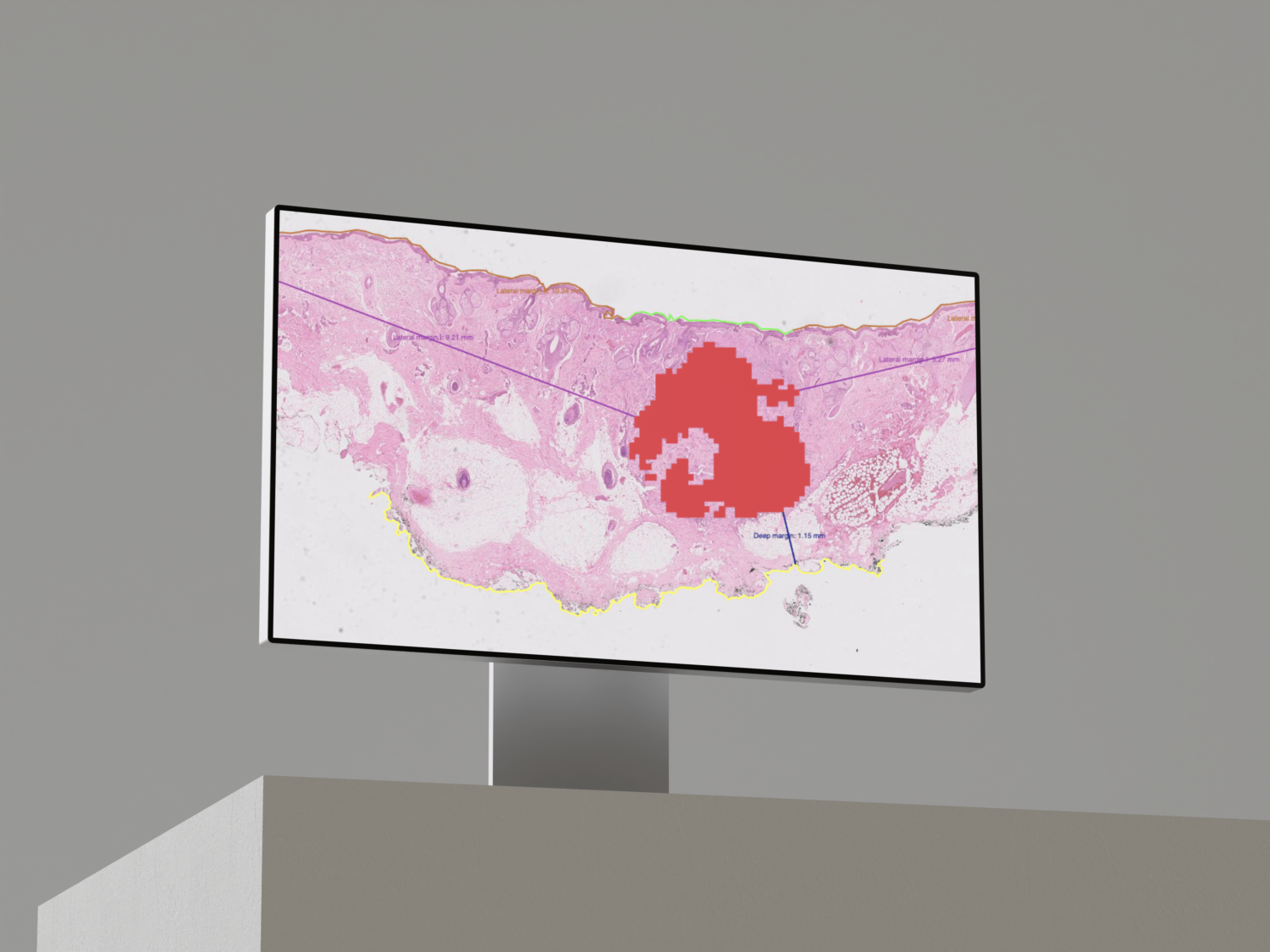
The Cleo Breast software automatically detects, on biopsies and surgical specimens, the main cancer biomarkers: Invasive and In Situ Carcinoma, Mitoses, Calcifications, and Lymph Node Metastases.
The clinical study conducted to evaluate the tool demonstrated its strong performance, both in terms of time savings and diagnostic accuracy. By using the tool’s features, physicians were able to reduce their overall diagnostic time by up to 15%.
“In the practice of my discipline, I was quickly confronted with the various complexities of breast cancer diagnosis, with time-consuming tasks that sometimes bring little added value. Our Cleo Breast tool automates some of these tasks and allows us to save valuable time, so that we can focus on aspects of diagnosis with greater added value,” explains Dr. Marie Sockeel, pathologist and co-founder of Primaa.
An IVDR certification that opens up new perspectives
With this new CE marking, Primaa confirms its position as a leader in Artificial Intelligence solutions applied to digital pathology.
This new milestone will enable the company to:
- Accelerate the deployment of Cleo Breast in European laboratories and hospitals,
- Prepare the international deployment of the solution, in particular in the U.S. market through FDA certification,
- And to progressively expand its range of tools to other organs, thanks to its next-generation AI models.
“We are proud to have obtained IVDR certification for Cleo Breast. This validates the scientific and clinical robustness of our solution and paves the way for its large-scale adoption in European laboratories,” explains Fanny Sockeel, CEO and co-founder of Primaa.
Cleo Breast had already been deployed in numerous French medical centers, such as Institut Curie, AP-HP, Hôpital Saint-Joseph, and others.
About Primaa:
Primaa develops artificial intelligence software capable of detecting various cancer biomarkers. The Cleo Breast (for breast) and Cleo Skin (for skin) tools support physicians in making their diagnoses, providing greater reliability and optimizing the time required for examination analysis. Co-founded in 2018 by Marie Sockeel, pathologist, together with Fanny Sockeel and Stéphane Sockeel, the company counts 25 employees.